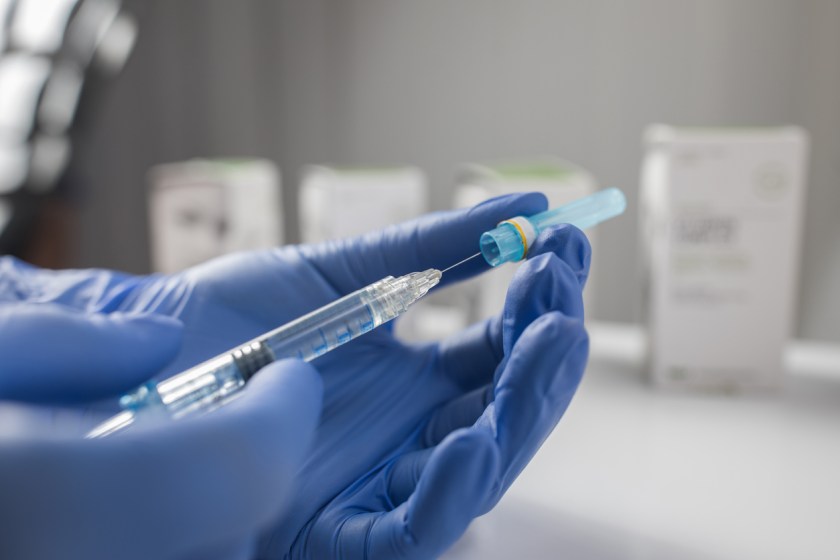
The petition specifically focuses on the risks associated with anti-wrinkle injections, calling for clearer labels and the removal of misleading promotional statements.
A consumer advocacy group in the US has recently filed a petition urging the U.S. Food and Drug Administration (FDA) to enhance safety warnings on anti-wrinkle injections, more commonly known as neuromodulators.
The petition, filed on behalf of Public Citizen, a non-for-profit consumer advocacy group, emphasises the need for a more robust black-box warning on both cosmetic and therapeutic anti-wrinkle injections, addressing the potential systemic iatrogenic botulism and related symptoms.
The current black box warning, as highlighted by Public Citizen, lacks clarity on the risks of systemic iatrogenic botulism, despite listing associated symptoms. It also neglects to provide information about antitoxin, the only specific therapy for all types of botulism.
Systemic iatrogenic botulism is a complication linked to the use of anti-wrinkle injections, wherein the toxin can spread beyond the injection sites, leading to progressive muscle paralysis or weakness. The severity of botulism may necessitate prompt administration of botulinum antitoxin to prevent further complications, including temporary muscle paralysis, hospitalisation, and potentially fatal outcomes.
The petition also targets three misleading promotional statements present in the labeling of anti-wrinkle injections, calling for their removal.
These statements, not found in labeling in other countries such as Canada and the United Kingdom, assert that “no definitive serious adverse events related to distant spread of the toxin’s effect have occurred with the use of recommended doses of these drugs for certain indications.”

Of these reports, 121 specified botulism as an adverse reaction, with 89 related to therapeutic uses and 32 to cosmetic uses. Additionally, 52% of the reports included signs or symptoms suggestive of iatrogenic botulism, consistent with the effect of anti-wrinkle injections.
As the FDA reviews the petition, the consumer advocacy group aims to address the potential risks associated with anti-wrinkle injections and provide consumers with more transparent information to make informed choices about their use.
As for Australia and the TGA, we have not been made aware of any similar cases to this, but will update this should we learn more.
Read the latest issue of SPA+CLINIC below:
There are 5 ways you can catch up with SPA+CLINIC
- Our quarterly print magazine, delivered to your door. Subscribe here.
- Our website, which is updated daily with its own completely unique content and breaking news.
- Our weekly newsletter – free to your inbox! Subscribe here.
- Our digital magazine – click here to view previous issues.
- Our social media – see daily updates on our Instagram, Facebook & Linkedin