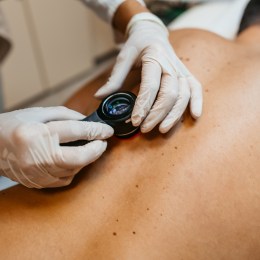
Will the COVID-19 vaccine change your treatment protocol? We spoke to Dr Giulia D’Anna.
Data from the FDA Moderna vaccine trial revealed that three of the 15,184 patients experienced facial swelling after receiving a single dose of the COVID-19 vaccine. One patient had dermal filler injected six months before the vaccine, and the other had it two weeks prior. The third patient’s injection window was unspecified.
However, all patients experienced localised swelling one to two days post-vaccination. There have also been overseas reports of delayed hypersensitive reactions in patients who received the COVID-19 vaccine outside of the clinical trials.
As Australia begins its phased roll-out of the vaccine, should injectors and patients be concerned? Dr Giulia D’Anna says no, and as an injector and dermal filler patient herself, she still will get the vaccine when it’s her time.
What is a Delayed Hypersensitive Reaction?
A delayed hypersensitive reaction (DHR) to dermal fillers is not a new phenomenon, and it’s not exclusively triggered by the COVID-19 vaccine either. Unlike an allergic reaction, it happens days, weeks or even months after the filler is administered.
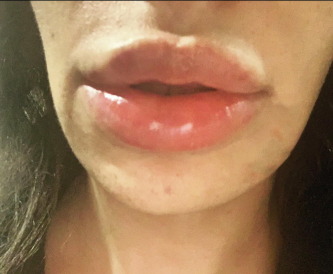
“This is therefore not recognised as an allergy, but rather an immune system reaction that occurs when there is another challenge in the body,” Dr Giulia said.
It’s common for patients to experience a delayed hypersensitive reaction during bacterial or viral illnesses or after other vaccines like the flu shot. Put simply, it’s the body attacking the filler as it mistakes it for an infection.
When we are sick, the body produces an immune response to fight off foreign cells. And while hyaluronic acid is naturally occurring in the body, hyaluronic acid filler is not. Instead, it’s genetically modified to increase longevity and ensure it isn’t rapidly broken down like naturally occurring HA.
“The manufacturing process of the HA is where the filler differs from our innate HA, and it is hypothesised that this processing of the filler creates the seed for delayed hypersensitivity as the T-lymphocytes see these structural chains as foreign when the immune system is triggered by influenza,” Dr Giulia said.
Filler or vaccine?
Luckily, this is a choice we won’t have to make. Most cases of delayed hypersensitive reactions settle down without intervention, and the reaction rates are still extremely low. The incidence of delayed hypersensitive reaction as a whole is 0.42 per cent, according to The Women’s Journal Of Dermatology. When it comes to DHRs and the COVID-19 vaccine, Dr Giulia expects data to grow as more people receive the vaccine.
But while there are currently no official regulations around the vaccine and dermal fillers, this information may alter your treatment protocols as more Australians receive the vaccine.
“There is some suggestion to wait four to eight weeks between filler treatment and vaccination so that as the immune system reacts to the vaccine, there is no further challenge from dermal filler being injected, and vice versa.
“As the vaccination program continues to roll out, I am sure that this will be an expanding area of reporting as more and more patients are vaccinated. However, in the early stages, the vaccine appears to have the same low reports of delayed hypersensitivity as in the general population that might experience these concerns when confronted with influenza,” she finishes.
DID YOU KNOW
There are 5 ways you can catch up with SPA+CLINIC?
- Our quarterly print magazine, delivered to your door. Subscribe here.
- Our website, which is updated daily with its own completely unique content and breaking news.
- Our weekly newsletter – free to your inbox! Subscribe here.
- Our digital magazine – click here to view previous issues.
- Our social media – see daily updates on our Instagram, Facebook & Linkedin