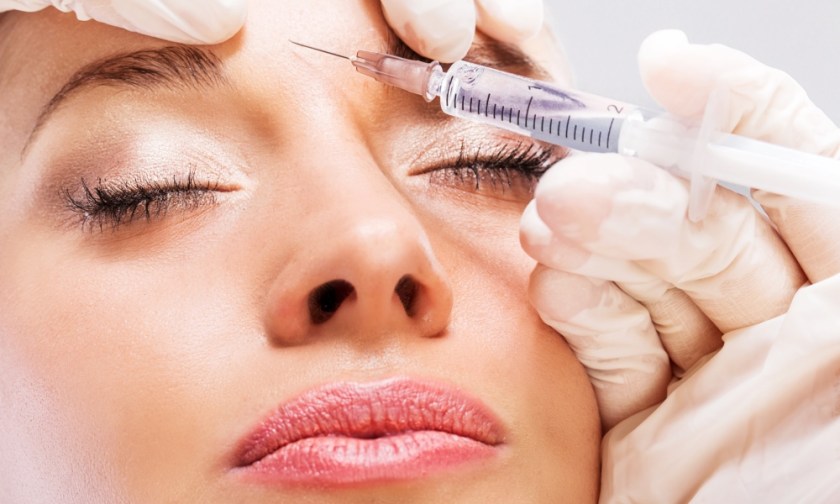
Revance Therapeutics has furrowed a few brows at Allergan, the home of Botox, with clinical trial results demonstrating that its new neuromodulator can reduce frown lines for around six months ‒ almost double the three to four months Botox achieves.
In an official statement, Revance said its next-generation neuromodulator, DaxibotulinumtoxinA (RT002), achieved “top-line results in alleviating glabella lines in two pivotal Sakura Phase 3 trials”.
The California-based bio-technology company tested the new injectable on the glabella lines (frown lines and wrinkles between brows) of more than 600 patients in the trials.
Both trials met the company’s primary goal of “delivering highly statistically significant improvement in reducing the severity of glabella lines” with 73.6 and 74 percent of patients respectively achieving at least “a two-point improvement from their baseline” at Week 4.
In addition, the patients received “highly statistically significant” results in glabellar reduction at every time point evaluated up to 24 weeks – and “the median duration for patients treated with RT002 to return to baseline wrinkle severity was nearly 27 weeks”.
Revance CEO Dan Browne, said the trial results prove it is scientifically and clinically possible to create a significantly longer-acting neuromodulator with duration of six months.
In addition, RT002, which is created using Revance’s proprietary peptide technology “eliminates the need for human- and animal-based components which carry a potential risk of transmitting pathogens”.
Treatment of glabella lines is the most popular aesthetic procedure for injectable neuromodulators such as Botox, Dysport and Xeomin, accounting for nearly a third of the US$3.6 billion sales in global neuromodulator sales last year.
According to Revance, patients and physicians alike identify “duration” as the most important attribute of an injectable aesthetic treatment.
At this stage the company is aiming to have RT002 on the market in 2020.